新闻中心
News Center
发布时间:2023-12-01
焦点提醒:厌氧氨氧化工艺快速启动策略和其微生物特征杨 瑞丽 1,2, 王 晓君 1, 吴 俊斌 1, 郭 焱 1,2, 张 召基 1 , 陈 少华 1,*1. 中国科学院城市情况研究所,城市污染物转化重点尝试室,厦门 3610212. 中国科学院年夜学,北京 100049 DOI10.12030/j.cjee.201804096 中图分类号 X703 文献标识码 A
 情况项目学报, 12(12): 3341-3350
情况项目学报, 12(12): 3341-3350摘 要 为切磋种泥投加和氮负荷晋升体例对厌氧氨氧化(anaerobic ammonia oxidation, anammox)工艺启动中微生物品貌和群落布局的影响,采纳先通俗活性污泥驯化后再接种anammox种泥的体例启动anammox工艺。成果注解在活性迟滞阶段投加anammox菌种能够快速启动anammox工艺。经由过程缩短水力逗留时候的体例增添氮负荷并能够避免间接提高进水氮浓度致使的基质毒性按捺,有益在到达更高的总氮去除负荷。不变运转时反映器的氮去除负荷达0.51 kg·(m3·d)−1,anammox菌基因品貌为4.92×109copies·g−1(以VSS计),占细菌总数的2.70%。启动阶段,反映器内微生物多样性逐步降落,检测到浮霉菌门中4个anammox菌属,以Candidatus Jettenia和Candidatus Kuenenia为首要anammox菌属。在接种污泥处在活性迟滞阶段时,连系提高进水氮浓度、缩短水力逗留时候和投加anammox菌种的体例能够快速启动anammox工艺。要害词 厌氧氨氧化(anammox);生物脱氮;微生物特征;快速启动Rapid start-up strategy and microbial characteristics of anammox processYANG Ruili1,2, WANG Xiaojun1, WU Junbin1, GUO Yan1,2, ZHANG Zhaoji1, CHEN Shaohua1,*1. Key Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China2. University of Chinese Academy of Sciences, Beijing 100049, China第一作者:杨瑞丽(1989—),女,博士研究生,研究标的目的:水污染节制手艺。E-mail:rlyang@iue.ac.cn*Corresponding author,E-mail:shchen@iue.ac.cn
Abstract In order to investigate the effects of seed sludge inoculation and increase mode for the influent nitrogen load on the microbial abundance and community in anaerobic ammonia oxidation (anammox) reactor during a start-up period, the anammox process was initiated by inoculating acclimated activated sludge with anammox seed sludge. Results showed that adding anammox strain at a lag stage was beneficial to the rapid start-up of pilot-scale reactor. An improved nitrogen removal rate (NRR) was achieved through shortening hydraulic retention time as well as increasing the influent nitrogen load, which was an effective means to avoid the inhibition of matrix toxicity caused by increasing the influent TN concentration. In the steady running phase, the NRR of 0.����APP51 kg·(m3·d)−1was realized, and the gene abundance of anammox bacteria reached 4.92×109copies·g−1(calculated by VSS), accounting for 2.70% of the total bacteria. In the start-up phase, the microbial diversity in the reactor gradually decreased, four anammox bacteria genus belonging toPlanctomyceteswere identified, and the dominant genus of functional bacteria wereCandidatus JetteniaandCandidatus Kuenenia. The anammox process was successfully start-up with short term by inoculating anammox seed sludge, raising influent nitrogen and shorting hydraulic retention time at the lag stage of inoculation sludge.keywords anaerobic ammonia oxidation (anammox);biological removal of nitrogen;microorganism characteristics;rapid start-up厌氧氨氧化(anaerobic ammonia oxidation, anammox)工艺因脱氮效力高、污泥产量低、无需外加碳源等劣势倍受接待,已被用在污泥消化液、味精废水、造酒废水和制药废水等的处置利用中[1-4]。但anammox菌具有发展速度慢、倍增时候长、情况敏感度高档缺点,是anammox工艺现实利用的主要瓶颈之一[5-6]。为了切磋anammox工艺快速启动的方式,前人做了年夜量研究,如考查反映器类型[7-8]、填料类型[9]、接种污泥源[10-12]和操作参数[13]等的影响。此中,污泥源研究多是基在接种污泥中间接投加anammox种泥的启动体例。唐崇俭等[10]经由过程接种硝化反硝化污泥、短程硝化污泥、厌氧絮体污泥和厌氧颗粒污泥并投加2%的anammox种泥,在255 d时,成功启动了中试(2.5 m3)anammox反映器。WANG等[11]接种尝试室低温收藏的anammox污泥,用时160 d,成功启动短程硝化厌氧氨氧化工艺。YE等[12]接种夹杂好氧、厌氧和同步部门硝化、厌氧氨氧化和反硝化污泥,在140 d时,氮去除负荷(nitrogen removal rate, NRR)达0.44 kg·(m3·d)−1。虽然将anammox污泥跟其他污泥夹杂后同时投加,增添了种泥中anammox菌的肇端品貌,但跟着菌体自溶阶段的运转,活性污泥解体必定会发生必然量的无机物而按捺anammox菌发展[2,12,14]。此时若起首投加通俗活性污泥,待启动进程的阻滞阶段再投加anammox种泥,能够有用避免无机物对其发展的按捺,且年夜年夜削弱了其他细菌跟anammox菌的合作。另外一方面,因为氨氮(NH4+-N)和亚硝氮(NO2−-N)对anammox菌都有迫害感化,假如纯真经由过程提高进水NH4+-N和NO2−-N浓度来增添进水氮负荷(nitrogen loading rate, NLR),则可能致使基质毒性按捺[2,15],而经由过程缩短水力逗留时候(hydraulic retention time, HRT)则可避免,且该体例少有研究。高通量测序手艺可同时获得上百万条DNA,并将其正确归类,是判定微生物的有用手段[16]。对anammox反映器进行功能菌定量阐发和微生物群落布局阐发,能够从功能菌群品貌和群落布局2个方面研究反映器启动进程微生物群落演变,为判定反映器成功启动与否供给间接根据[16-17],并为反映器的不变运转和调控供给科学根据。本研究摸索采纳先通俗活性污泥驯化后再接种anammox种泥的体例快速启动anammox工艺,并采取先慢慢提高进水总氮(total nitrogen, TN)浓度后缩短HRT的方式提高进水NLR。同时操纵Illumina Miseq测序和及时定量聚合酶链式反映(polymerase chain reaction, PCR)方式,阐发了启动进程中污泥微生物群落转变,为深切熟悉anammox工艺的启动历程供给根据。
1 材料和方式
1.1 尝试装配尝试采取升流式厌氧氨氧化反映器,尝试装配示企图和反映器照片见图1。反映器有用容积100 L,顶部加盖,由螺钉和胶条密封,并预留有排气孔。距底部2 cm和顶部10 cm处罚别设有进水口和出水口,中部和底部设有污泥采样口。反映器内部装填鲍尔环填料。内设回流装配,强化反映器内溶液夹杂,防止进水端反映物浓渡过高,也起到避免进水口梗塞的感化。反映器进水pH经0.1 mol·L−1盐酸调理至7.2摆布,外部包裹电加热带以包管内部恒温(35±2) °C,避光运转,保持anammox菌适合的发展情况。
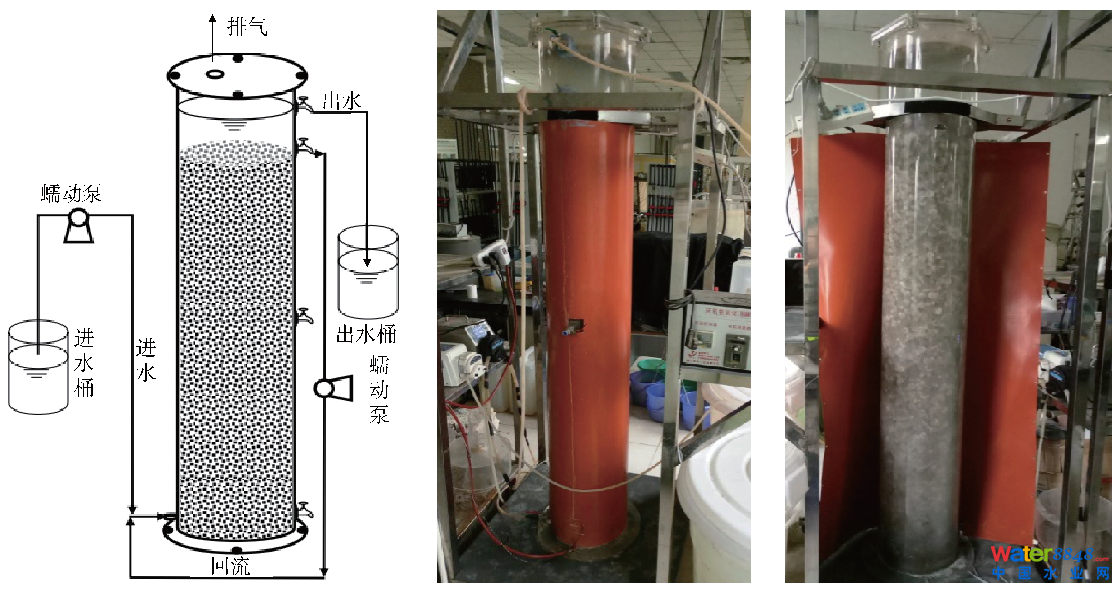 图1尝试装配示企图和反映器照片Fig. 1Schematic diagram and pictures of experimental equipment
图1尝试装配示企图和反映器照片Fig. 1Schematic diagram and pictures of experimental equipment1.2 尝试前提和运转策略尝试用水为人工配制的摹拟废水,具体构成见表1。此中,进水NH4+-N和NO2−-N浓度均为30~500 mg·L−1,NO3−-N浓度保持在100 mg·L−1之内。别的,微量元素配方为ZnSO4·7H2O 0.43 g·L−1、CoCl2·6H2O 0.24 g·L−1、MnCl2·4H2O 0.99 g·L−1、CuSO4·5H2O 0.25 g·L−1、NaMoO4·2H2O 0.043 g·L−1、NiCl2·6H2O 0.20 g·L−1、KH2PO420 g·L−1、H3BO30.014 g·L−1,并插手EDTA 20 g·L−1以增进微量元素的消融。收集进出水水样经0.45 μm滤膜过滤后,别离采取纳氏试剂法、N-(1-萘基)乙二胺分光光度法和紫外分光光度法测定样品中的NH4+-N、NO2−-N和NO3−-N[18]。pH采取便携式pH计(FG2-FK, METTLER TOLEDO, USA)测定;污泥样品MLSS、MLVSS均按尺度方式[19]测定。
 表1摹拟废水成份Table 1Chemical composition of synthetic wastewateranammox工艺启动进程首要分为4个阶段,别离是Ⅰ菌体自溶、Ⅱ活性迟滞、Ⅲ活性提高和Ⅳ活性不变阶段[7,20]。本研究采取2阶段接种体例启动anammox反映器。起首接种80 L取自厦门市某污水处置厂二沉池的活性污泥,使反映器中ρ(SS)=47.05 g·L−1,ρ(VSS)=13.19 g·L−1。比及污泥处在anammox活性迟滞阶段时(第60 天),投加已培育10个月具有anammox活性的种泥(泥色微红,ρ(SS)=26.69 g·L−1,ρ(VSS)=13.52 g·L−1),接种量为20 L。经由过程慢慢提高进水TN浓度(41~116 d)和缩短HRT(117~172 d)来提高反映器NLR,具体运转参数见表2。
表1摹拟废水成份Table 1Chemical composition of synthetic wastewateranammox工艺启动进程首要分为4个阶段,别离是Ⅰ菌体自溶、Ⅱ活性迟滞、Ⅲ活性提高和Ⅳ活性不变阶段[7,20]。本研究采取2阶段接种体例启动anammox反映器。起首接种80 L取自厦门市某污水处置厂二沉池的活性污泥,使反映器中ρ(SS)=47.05 g·L−1,ρ(VSS)=13.19 g·L−1。比及污泥处在anammox活性迟滞阶段时(第60 天),投加已培育10个月具有anammox活性的种泥(泥色微红,ρ(SS)=26.69 g·L−1,ρ(VSS)=13.52 g·L−1),接种量为20 L。经由过程慢慢提高进水TN浓度(41~116 d)和缩短HRT(117~172 d)来提高反映器NLR,具体运转参数见表2。 表2反映器各阶段运转参数Table 2Reactor operation parameters of each phase
表2反映器各阶段运转参数Table 2Reactor operation parameters of each phase1.3 尝试装配DNA提取、及时定量PCR阐发与多样性测定
1.3.1 DNA提取别离在工艺启动0、59、76、83、94、104、116、126、138、156和172 d时,收集污泥夹杂液。经30 min静沉后,称取500 mg污泥样品,利用FastDNA™ SPIN Kit for Soil(LLC, MP Biomedicals, USA)提取试剂盒,按其操作步调提取污泥样品中总DNA。DNA经1%琼脂糖凝胶电泳和Nanodrop(ND1000, Gene Company Limited, China)检测其纯度和浓度落后行及时定量PCR尝试,并从当选取0、59、104和172 d的DNA样品进行Illumina高通量测序。
1.3.2 及时定量PCR阐发及时定量PCR是一种能够正确定量功能基因拷贝数,进而计较功能菌品貌的尝试方式。本尝试采取Roche LightCycler®480 Ⅱ(Roche Diagnostics Ltd., Rotkreuz, Swltzerland)及时荧光定量系统进行品貌阐发,采取20 μL反映系统,具体设置装备摆设为:SYBR Green Ⅰ Master(LightCycler®480, mannheim, Germany)10 μL;前后引物各0.8 μL;质粒或DNA样品1 μL;去离子水 7.4 μL。此中,全细菌定量引物为通用引物341F:534R[21],而anammox菌采取特同性引物Amx808F(5'-ARC YGT AAA CGA TGG GCA CTA A-3')和Amx1040R (5'-CAG CCA TGC AAC ACC TGT RAT A-3')[21-22]。及时定量PCR运转法式为3步法:95 °C预变性5 min,35个轮回(95 °C变性30 s,45 °C退火30 s,72 °C延长30 s),72 °C终延长10 min,最初进行消融曲线阐发,并计较anammox菌的绝对品貌和其在总细菌中的占比。
1.3.3 Illumina高通量测序取样品16S rRNA基因中的V4~V5区,选用引物515F (5′-GTG CCA GCM GCC GCG G-3′)和907R (5′-CCG TCA ATT CMT TTR AGT TT-3′)进行扩增,扩减产物经2%琼脂糖凝胶电泳质控,并均一化后,进行Miseq文库建立,并采取Illumina Miseq测序平台对样品进行高通量测序。源数据经质控处置后,挑选高质量数据用MOTHUR软件法式进行阐发。
2 成果与会商
2.1 厌氧氨氧化工艺启动进程脱氮机能转变图2为anammox反映器启动进程各氮物资浓度转变。反映器运转早期1~40 d为阶段Ⅰ,出水NH4+-N和NO2−-N浓度均高在进水,为微生物的自消融体阶段[5,20]。该阶段出水NO2−-N浓度逐步降落,多是反映器中共存有氨氧化菌(ammonia oxidizing bacteria, AOB)和反硝化菌(denitrification bacteria, DNB),此中AOB操纵进水中微量的消融氧将NO2−-N转化为NO3−-N,DNB则将NO3−-N和NO2−-N还原为N2,使NO2−-N浓度降落[5,23]。CHAMCHOI等[7]、GUO等[24]和BI等[25]研究注解,工艺启动早期,反映器内部微生物呈现菌体细胞自溶,释放出年夜量的无机物和NH4+-N,同时无机氮被分化,为异养微生物供给碳源,使出水NH4+-N和NO2−-N浓度高在进水,与本研究成果分歧。反映器运转41~59 d为阶段Ⅱ,此时出水NH4+-N和NO2−-N同步去除,注解anammox菌已显示出必然活性[5,20,26]。ΔNO2−-N/ΔNH4+-N值平均为1.14,略低在STROUS等[27]报导的值,且出水NO3−-N浓度低在进水,申明反映器内仍有一部门DNB操纵残留无机质做电子供体还原NO3−-N为N2[28]。
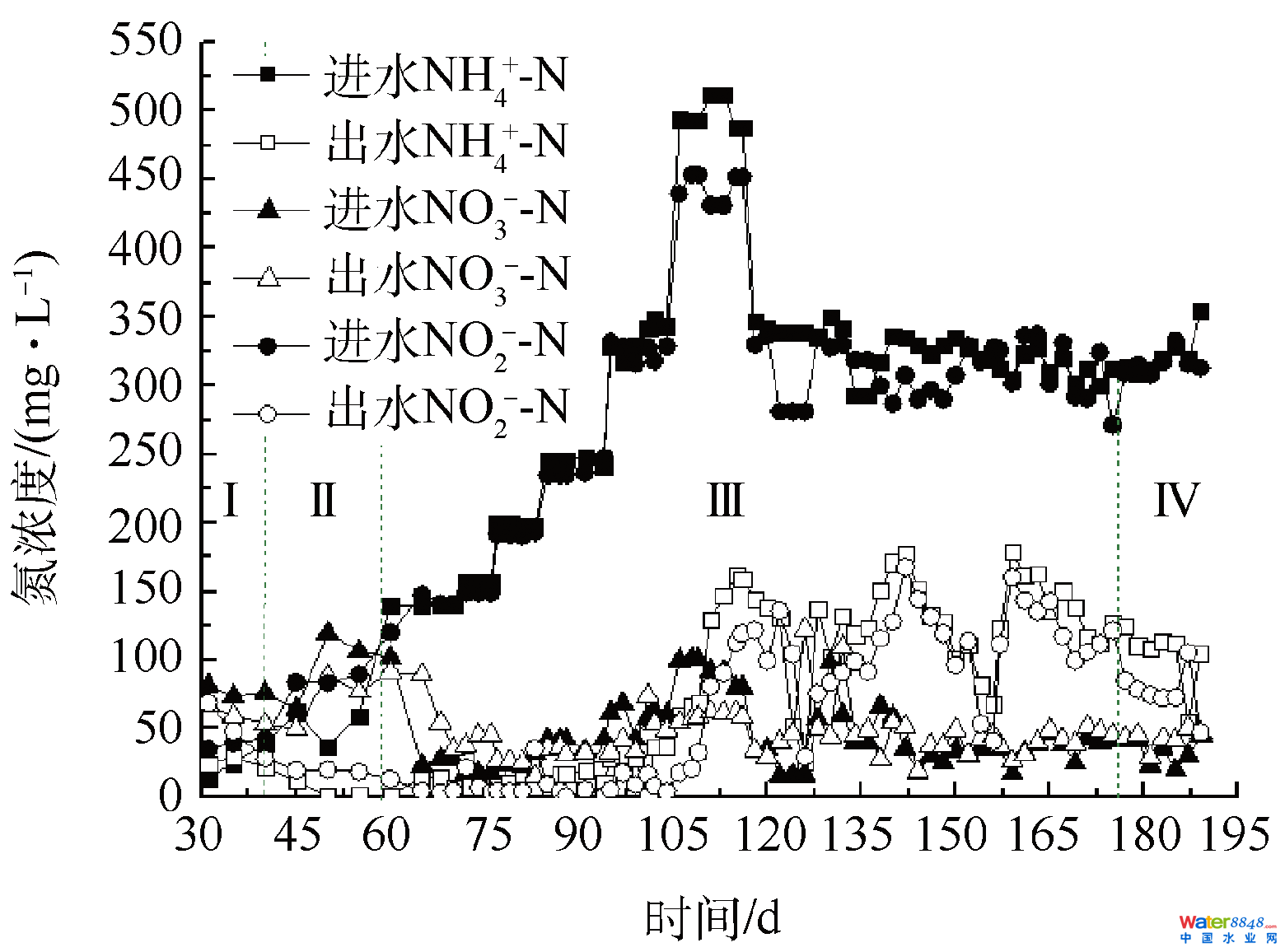 图2中试厌氧氨氧化工艺启动中进出水氮浓度转变Fig. 2Nitrogen variation of influent and effluent during start-up of anammox process in a pilot-scale reactor反映器运转第60 天,再接种20 L富含anammox菌的种泥,然后以提高进水氮浓度和缩短HRT的体例提高反映器NLR,此阶段为阶段Ⅲ。连系试厌氧氨氧化工艺启动中进出水氮浓度转变(图2)和厌氧氨氧化工艺启动中脱氮效力、NLR、NRR和ΔNO2−-N/ΔNH4+-N比值的转变(图3)可知,接种anammox种泥起到了不变反映器脱氮机能的感化,虽然在接种anammox种泥的同时,提高了进水TN浓度,也可以使反映器在10 d内TN去除率从64.70%晋升至84.77%,增进了反映器的快速启动[29]。这是由于颠末阶段Ⅰ和Ⅱ的培育后,反映器内可降解的无机碳源年夜幅下降,与anammox菌合作的异养微生物显著削减,此时添加有anammox活性的种泥可以使反映器内anammox菌品貌敏捷增添,利在anammox活性的快速晋升[30]。唐崇俭等[10]和WANG等[11]指出anammox种泥的添加是anammox工艺脱氮机能加强的要害。
图2中试厌氧氨氧化工艺启动中进出水氮浓度转变Fig. 2Nitrogen variation of influent and effluent during start-up of anammox process in a pilot-scale reactor反映器运转第60 天,再接种20 L富含anammox菌的种泥,然后以提高进水氮浓度和缩短HRT的体例提高反映器NLR,此阶段为阶段Ⅲ。连系试厌氧氨氧化工艺启动中进出水氮浓度转变(图2)和厌氧氨氧化工艺启动中脱氮效力、NLR、NRR和ΔNO2−-N/ΔNH4+-N比值的转变(图3)可知,接种anammox种泥起到了不变反映器脱氮机能的感化,虽然在接种anammox种泥的同时,提高了进水TN浓度,也可以使反映器在10 d内TN去除率从64.70%晋升至84.77%,增进了反映器的快速启动[29]。这是由于颠末阶段Ⅰ和Ⅱ的培育后,反映器内可降解的无机碳源年夜幅下降,与anammox菌合作的异养微生物显著削减,此时添加有anammox活性的种泥可以使反映器内anammox菌品貌敏捷增添,利在anammox活性的快速晋升[30]。唐崇俭等[10]和WANG等[11]指出anammox种泥的添加是anammox工艺脱氮机能加强的要害。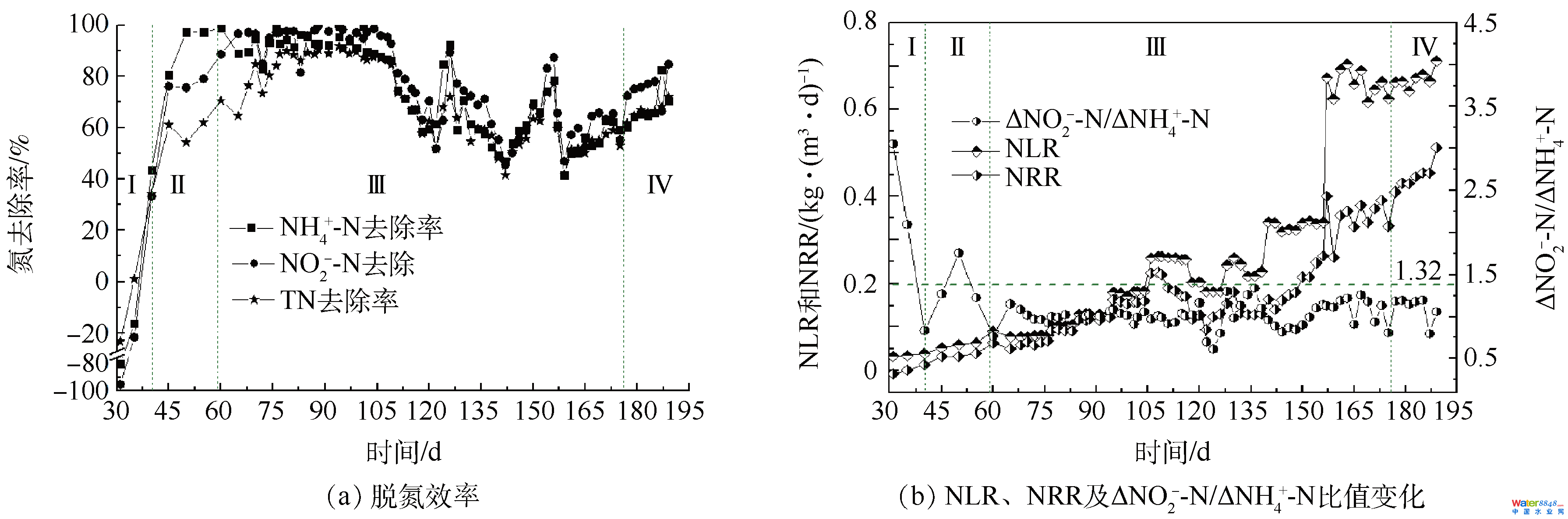 图3厌氧氨氧化工艺启动中脱氮效力、NLR、NRR和ΔNO2−-N/ΔNH4+-N比值转变Fig. 3Nitrogen removal performance of anammox reactor, including nitrogen removal efficiency, NLR, NRR and Δnitrite/Δammonium当进水TN浓度≤700 mg·L−1(60~104 d)时,固然进水TN浓度延续增添,但反映器出水氮浓度并没有较着转变,反映器NRR达0.16 kg·(m3·d)−1。当进水TN浓度达1 000 mg·L−1(105~116 d)时,TN去除率在12 d内由87.51%降落至66.89%,NRR较105 d时降落了24.16%。第116天,出水NH4+-N和NO2−-N浓度别离为104 d时的4.26和26.67倍,这多是由于太高的NO2−-N浓度按捺了anammox菌的活性。曹雁等[17]也发觉,当进水NH4+-N和NO2−-N浓度别离到达330 mg·L−1和430 mg·L−1时,反映器出水水质忽然恶化。研究注解,NO2−-N对anammox菌的影响高在NH4+-N[31],NH4+-N浓度低在1 000 mg·L−1时不会按捺anammox菌活性,而NO2−-N高在280 mg·L−1时便会发生按捺[32],DAPENA-MORA等[33]也注解350 mg·L−1的NO2−-N会按捺anammox菌50%的活性。为确保反映器快速恢复并不变运转,在反映器运转117~126 d时代,将进水TN浓度降至700 mg·L−1,同时HRT由本来的4 d缩短为3.5 d,NLR也响应地由0.25 kg·(m3·d)−1降至0.19 kg·(m3·d)−1。因为进水基质浓度下降对anammox菌的按捺感化消除,反映器anammox活性快速恢复,10 d内NH4+-N、NO2−-N和TN去除率别离由67.27%、73.55%和66.89%快速升至92.41%、89.47%和72.05%。反映器运转127~172 d,HRT由3.5 d缩短至2 d,进而缩短至1 d,每次缩短HRT,反映器出水水质都有一个恶化进而恢复的进程,注解功能菌群慢慢顺应着发展情况的转变[34]。第172天,NH4+-N、NO2−-N和TN去除率和NRR别离达60.82%、65.16%、57.70%和0.38 kg·(m3·d)−1。与提高氮浓度对反映器脱氮机能的影响比拟,缩短HRT对提高反映器脱氮机能感化更年夜。研究注解,缩短HRT一方面会加快夹杂反映基质,另外一方面会加年夜系统水流剪切力,这有益在anammox反映器的运转[35-36]。阶段Ⅳ(173~189 d)为anammox反映器的活性不变阶段,终究NH4+-N、NO2−-N和TN去除率别离不变在76.50%、76.45%和70.04%摆布,NRR达0.51 kg·(m3·d)−1。汪瑶琪等[34]在小试19.6 L的反映器中,将好氧污泥和anammox种泥以2:1的体积比夹杂启动anammox工艺,耗时157 d,使NRR达0.44 kg·(m3·d)−1,本研究成果较其略有劣势。JIN等[26]提出以NRR 达0.5 kg·(m3·d)−1为anammox反映器成功启动的尺度,注解中试anammox工艺已成功启动。在反映器不变运转时,ΔNO2−-N:ΔNO3−-N:ΔNH4+-N平均为1.16:0.06:1,低在厌氧氨氧化反映化学计量式,申明仍有必然的DNB共存[26]。
图3厌氧氨氧化工艺启动中脱氮效力、NLR、NRR和ΔNO2−-N/ΔNH4+-N比值转变Fig. 3Nitrogen removal performance of anammox reactor, including nitrogen removal efficiency, NLR, NRR and Δnitrite/Δammonium当进水TN浓度≤700 mg·L−1(60~104 d)时,固然进水TN浓度延续增添,但反映器出水氮浓度并没有较着转变,反映器NRR达0.16 kg·(m3·d)−1。当进水TN浓度达1 000 mg·L−1(105~116 d)时,TN去除率在12 d内由87.51%降落至66.89%,NRR较105 d时降落了24.16%。第116天,出水NH4+-N和NO2−-N浓度别离为104 d时的4.26和26.67倍,这多是由于太高的NO2−-N浓度按捺了anammox菌的活性。曹雁等[17]也发觉,当进水NH4+-N和NO2−-N浓度别离到达330 mg·L−1和430 mg·L−1时,反映器出水水质忽然恶化。研究注解,NO2−-N对anammox菌的影响高在NH4+-N[31],NH4+-N浓度低在1 000 mg·L−1时不会按捺anammox菌活性,而NO2−-N高在280 mg·L−1时便会发生按捺[32],DAPENA-MORA等[33]也注解350 mg·L−1的NO2−-N会按捺anammox菌50%的活性。为确保反映器快速恢复并不变运转,在反映器运转117~126 d时代,将进水TN浓度降至700 mg·L−1,同时HRT由本来的4 d缩短为3.5 d,NLR也响应地由0.25 kg·(m3·d)−1降至0.19 kg·(m3·d)−1。因为进水基质浓度下降对anammox菌的按捺感化消除,反映器anammox活性快速恢复,10 d内NH4+-N、NO2−-N和TN去除率别离由67.27%、73.55%和66.89%快速升至92.41%、89.47%和72.05%。反映器运转127~172 d,HRT由3.5 d缩短至2 d,进而缩短至1 d,每次缩短HRT,反映器出水水质都有一个恶化进而恢复的进程,注解功能菌群慢慢顺应着发展情况的转变[34]。第172天,NH4+-N、NO2−-N和TN去除率和NRR别离达60.82%、65.16%、57.70%和0.38 kg·(m3·d)−1。与提高氮浓度对反映器脱氮机能的影响比拟,缩短HRT对提高反映器脱氮机能感化更年夜。研究注解,缩短HRT一方面会加快夹杂反映基质,另外一方面会加年夜系统水流剪切力,这有益在anammox反映器的运转[35-36]。阶段Ⅳ(173~189 d)为anammox反映器的活性不变阶段,终究NH4+-N、NO2−-N和TN去除率别离不变在76.50%、76.45%和70.04%摆布,NRR达0.51 kg·(m3·d)−1。汪瑶琪等[34]在小试19.6 L的反映器中,将好氧污泥和anammox种泥以2:1的体积比夹杂启动anammox工艺,耗时157 d,使NRR达0.44 kg·(m3·d)−1,本研究成果较其略有劣势。JIN等[26]提出以NRR 达0.5 kg·(m3·d)−1为anammox反映器成功启动的尺度,注解中试anammox工艺已成功启动。在反映器不变运转时,ΔNO2−-N:ΔNO3−-N:ΔNH4+-N平均为1.16:0.06:1,低在厌氧氨氧化反映化学计量式,申明仍有必然的DNB共存[26]。2.2 厌氧氨氧化反映器启动进程anammox菌品貌转变
2.2.1 污泥色彩转变由图4可知,初始接种时反映器内污泥色彩为墨黑色。经59 d厌氧驯化后,初显anammox活性的污泥色彩转为深灰色。跟着anammox活性提高污泥色彩慢慢变红,终究呈红棕色。MOLINUEVO等[37]研究发觉,anammox菌因内部含有年夜量血红素C而显现红色,在本研究中,泥色逐步变红,进一步注解中试anammox工艺已成功启动。
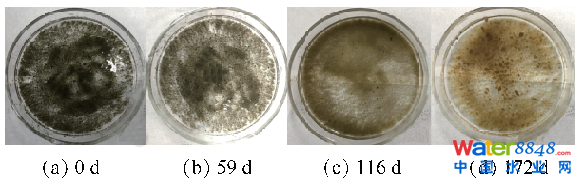 图4厌氧氨氧化反映器启动进程污泥色彩转变Fig. 4Variation of sludge color during anammox process start-up phase
图4厌氧氨氧化反映器启动进程污泥色彩转变Fig. 4Variation of sludge color during anammox process start-up phase2.2.2 anammox菌品貌转变由图5可知,跟着anammox反映器运转时候耽误,anammox菌基因品貌逐步增添,终究品貌达4.92×109copies·g−1(以VSS计)。CHEN等[29]别离将反硝化和anammox种泥以3:1的体积比夹杂启动anammox工艺,成功启动时anammox菌基因品貌为4.13×109copies·g−1。YIN等[9]采取添加氧化石墨烯的体例,增进anammox工艺的启动,启动成功时anammox菌基因品貌为1.84×109copies·g−1。本研究成果高在两者成功启动时的anammox菌基因品貌。16S rRNA基因品貌无显著性转变,一直保持在(2.03±2)×1011copies·g−1,anammox菌占16S rRNA的百分比由开初的0.01%渐增至2.70%。反映器运转1~59 d,anammox菌基因品貌由接种时的7.31×107copies·g−1增至5.62×108copies·g−1,增添了7.7倍。同时,16S rRNA基因品貌由5.20×1011copies·g−1降落至1.09×1011copies·g−1,注解anammox菌最先慢慢成为首要菌群之一[16]。颠末60~172 d的活性提高,anammox菌占16S rRNA的百分比由之前的0.51%增添至2.70%。CHEN等[29]接种常规反硝化污泥启动anammox反映器,终究anammox菌占16S rRNA的百分比为3%摆布。HU等[38]以接种水稻土启动SBR型anammox反映器,18个月后,anammox菌占16S rRNA的百分比约为5.3%。对照文献研究成果,从anammox菌绝对品貌和相对品貌两方面均注解anammox反映器已成功启动,且用时较HU等[38]缩短了6个月。
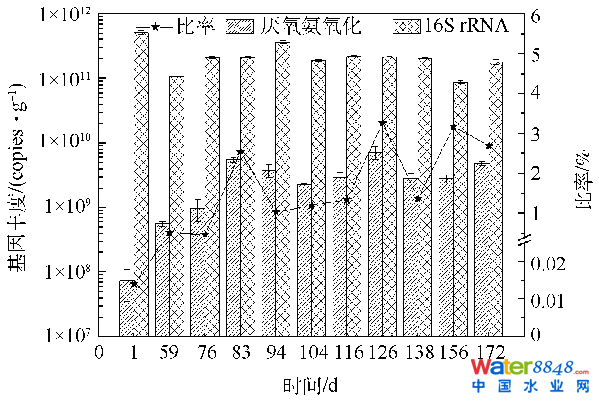 图5厌氧氨氧化工艺启动中anammox菌基因品貌转变Fig. 5Changes in gene abundance of anammox bacteria during start-up of anammox process
图5厌氧氨氧化工艺启动中anammox菌基因品貌转变Fig. 5Changes in gene abundance of anammox bacteria during start-up of anammox process2.3 厌氧氨氧化反映器快速启动中菌群多样性的转变
2.3.1 厌氧氨氧化反映器启动中Alpha多样性阐发厌氧氨氧化反映器启动进程中Alpha多样性指数的转变见表3。从表3能够看出,厌氧氨氧化反映器启动中高通量测序的4个样品笼盖率指数均高在0.99,注解检测成果根基涵盖所有微生物的16S rRNA基因[34]。表3中不雅察物种、Chao1和ACE指数暗示样品中物种数目的几多,值越年夜,物种越丰硕;Shannon、Simpson和PD whole tree指数暗示微生物群落构成的复杂度,其值越高,复杂度越高[39-40]。随反映器NLR的提高,6种多样性指数整体呈降落的趋向,注解跟着驯化时候的耽误,反映器内微生物丰硕度和复杂度下降。
 表3厌氧氨氧化反映器启动进程中Alpha多样性指数转变Table 3Alpha diversity analysis during start-up of anammox process in a pilot-scale reactor
表3厌氧氨氧化反映器启动进程中Alpha多样性指数转变Table 3Alpha diversity analysis during start-up of anammox process in a pilot-scale reactor2.3.2 门程度物种品貌阐发由图6可知,反映器运转时代相对品貌较高的菌群首要有变形菌门(Proteobacteria)、绿菌门(Chlorobi)、绿弯菌门(Chloroflexi)、拟杆菌门(Bacteroidetes)、装甲菌门(Armatimonadetes)、Parcubacteria门、浮霉菌门(Planctomycetes)等。不管是在废水处置、厌氧消化仍是泥土中,变形菌门均为最多见的贯串一直的劣势菌群[38,41-42],其品貌可在10.3%~55.5%间波动,为首要的脱氮功能菌群之一。本研究中,变形菌门相对品貌随反映器NLR的晋升,呈先降后升的趋向。反映器运转0~104 d,由驯化早期的48.87%逐步降落至22.92%,后在172 d时回升至41.02%,与CONNAN等[43]在anammox驯化进程中报导的变形菌门的转变趋向不异。绿弯菌门、拟杆菌门和装甲菌门是常见的可与anammox菌共存的菌群[44],同Parcubacteria门一路,其相对品貌均随驯化而先下降后上升,转变规模别离为15.48%~23.97%、1.80%~7.56%、1.47%~6.65%和0.26%~4.46%。CAO 等[16]对启动成功的anammox-UASB反映器内的污泥样品进行了群落布局阐发,发觉变形菌门、绿弯菌门、拟杆菌门、硝化螺旋菌门、酸杆菌门、装甲菌门、绿菌门和浮霉菌门为首要菌门,变形菌门和绿弯菌门占有比值别离为27.07%~32.24%和25.77%~29.14%,并指出绿弯菌门和拟杆菌门与污泥颗粒的构成相干。CONNAN等[43]研究报导,anammox反映器内微生物菌门首要为变形菌门、拟杆菌门、硬壁菌门、绿弯菌门、绿菌门、酸杆菌门和浮霉菌门,变形菌门品貌高达55.9%,绿弯菌门和绿菌门是anammox反映器内常见菌门。浮霉菌门为首要的自养脱氮功能菌群,此刻已知的具有厌氧氨氧化功能的微生物均属在浮霉菌门[45]。其转变趋向与变形菌门相反,随反映器NLR的晋升,浮霉菌门相对品貌由0 d时的1.34%逐步升高至172 d的3.64%,注解anammox菌获得了必然的富集。绿菌门相对品貌的转变趋向与浮霉菌门转变趋向分歧。本研究成果与CAO 等[16]和CONNAN等[43]的成果附近,注解anammox颗粒污泥的构成可能受益在绿弯菌门、拟杆菌门、装甲菌门、绿菌门和Parcubacteria门品貌的增添。
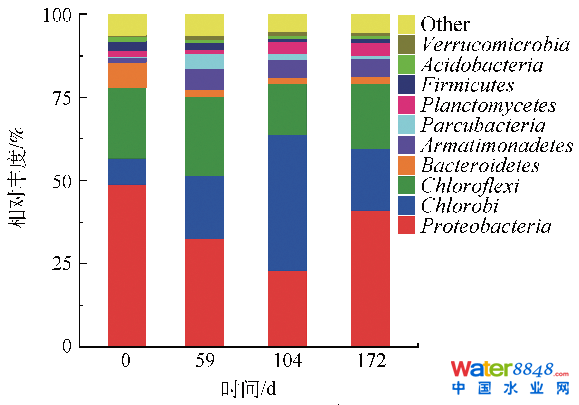 图6中试anammox反映器启动进程中首要菌群转变(门程度)Fig. 6Variation of main microbial flora at a phylum level during start-up of anammox process in a pilot-scale reactor
图6中试anammox反映器启动进程中首要菌群转变(门程度)Fig. 6Variation of main microbial flora at a phylum level during start-up of anammox process in a pilot-scale reactor2.3.3 浮霉菌门中属程度物种品貌阐发为进一步领会anammox反映器启动中内部anammox菌的转变,拔取浮霉菌门进行属程度的物种品貌阐发。浮霉菌门首要有I-8、Candidatus Jettenia、Candidatus Kuenenia、SM1A02和Planctomyces等。在已知的具有厌氧氨氧化能力的6个属[45]中,本中试反映器检测到4个,别离是Candidatus Jettenia、Candidatus Kuenenia、Candidatus Brocadia和Candidatus Anammoximicrobium。由表4可知,中试anammox反映器内Candidatus Jettenia和Candidatus Kuenenia为anammox的首要菌属。同时,二者也是污水处置厂和年夜范围anammox反映器中常见的2个属[46]。以后随反映器内NLR逐步增年夜,Candidatus Jettenia和Brocadia属品貌呈先增添后降落的趋向,反映器运转59~104 d,别离由13.50%和1.50%逐步增至39.51%和1.72%,后在172 d时降至5.26%和0.20%。而Candidatus Kuenenia属品貌随NLR增年夜而逐步增添,第172天时Kuenenia属品貌为第59 天时品貌的3.33倍,而Candidatus Anammoximicrobium属品貌随NLR增年夜而逐步下降,第172 天时已检测不到,多是由于Candidatus Kuenenia的发展速度、亚硝酸盐亲和力和对水量变化的反抗力比其他anammox菌属强[47-48]。运转时代,anammox菌占总细菌的百分比逐步增添,最高可达1.65%(Jettenia+Kuenenia+Brocadia+Anammoximicrobium)。及时定量PCR成果显示,在172 d时,anammox反映器中anammox菌的相对品貌为2.7%,与Illumina高通量测序成果接近,进一步印证了anammox工艺已快速启动。
 表4浮霉菌门中部门菌属的相对品貌转变Table 4Changes in relative abundance of some genus of planctomycetes
表4浮霉菌门中部门菌属的相对品貌转变Table 4Changes in relative abundance of some genus of planctomycetes3 结论1)在接种污泥处在anammox活性迟滞阶段时,投加富含anammox菌的种泥能够快速启动anammox反映器,且在anammox活性提高阶段,经由过程缩短HRT可以或许避免间接提高进水TN浓度带来的基质毒性按捺,能够更年夜水平上提高反映器氮去除负荷,启动成功后,anammox反映器终究NRR达0.51 kg·(m3·d)−1。2)在启动进程中,接种污泥色彩由墨黑色逐步变成红棕色,anammox菌品貌随NLR的增添逐步上升,终究基因品貌达4.92×109copies·g−1(以VSS计),占总细菌的2.70%。3)在工艺启动阶段,anammox反映器内微生物复杂度逐步下降,在浮霉菌门中检测到4个anammox菌属:Candidatus Jettenia、Candidatus Kuenenia、Candidatus Brocadia和Candidatus Anammoximicrobium属,占测序总数的1.65%,Candidatus Jettenia和Candidatus Kuenenia是反映器中首要的anammox菌属。
参考文献